Enoxaparin Sodium
CAS No.: 679809-58-6
Specification
| Enoxaparin Sodium | ||||
| Source | Intestinorum mucosa de porcos | |||
| Qualis vexillum | EP | |||
| characteribus | Aspectus | / | albus vel fere albus;hygroscopic pulveris | |
| solubility | / | gratis solutum in aqua | ||
| idem | Et Spectra Exhibit Maxima ad CCXXXI ± 2nm | The13C NMR spectro adeptus est similis, qui adeptus est conveniens speciei enoxaparin sodium CRS | ||
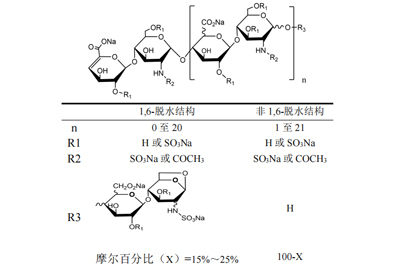
| spectris similes | XV -25% afferentem 1,6-anhydro structuram ad redigo finem eorum chain15% - XXV% of components ferre de 1,6-Anhydro structuram ad reducing finem suum torquem | |||
| Mw : 3800 -5000Da, M: 12.0%-20.0%, M≥8000:NMT 18.0%, M2000-8000 | Mw : 3800 -5000Da, M M2000-8000 | |||
| Metus requisitis pro Sodium chemicis identificatio probat | Prosequitur tium sodium | |||
| Solutio specie | / | clear;NMT: intensio VI " | ||
| Imprimis Absorbance | 14.0-20.0 (ex exaruit basis) | 14.0-20.0 (ex sicco basis), ad 231nm . determinatum | ||
| pH | ||||
| Benzyl alcohol | ≤ 0.1% | ≤ 0.1% | ||
| Ratio molaris sulfatis carboxylata | ||||
| Nitrogen | 1.8% -2.5% (ex exaruit basis) | |||
| Natrium | 11.3% -13.5% (ex exaruit basis) | 11.3% -13.5% (ex exaruit basis) | ||
| Damnum in siccitate | ||||
| ≤0.01EU/U | ≤0.01EU/IU | |||
| Assay | 90-125 III/mg (in exaruit fundamentum) | 90-125 III/mg (in exaruit fundamentum) | ||
| 20.0-35.0 IU/mg (ex sicco basis) | 20.0-35.0 IU/mg (ex sicco basis) | |||
Indicium
1. Praeventionis morborum venarum thromboembolicorum (venarum thrombosis praeventionis), praesertim relate ad chirurgicam orthopaedicam vel generalem.
2. Curatio embolismi venae altae existendi, cum vel sine embolismo pulmonis, cum lenis clinicis symptomatibus, exclusis embolismo pulmonali, quae chirurgicam vel thrombolyticam requirit.
3. Curatio angina pectoris instabilis et infarctus myocardialis non-Q unda, cum aspirino coniuncta.
4. usus est in cardiopulmonary bypass hemodialysis ne thrombosis.
CAS No.: 9041-08-1 (Pondus hypotheticum Minimum heparin)
CAS No.: 37270-89-6.