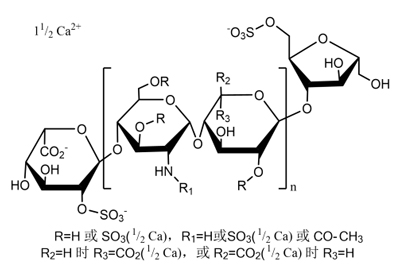
Nadroparin Calcium
CAS No.: 37270-89-6.
Specification
| Nadroparin calcium | ||
| Source | Intestinorum mucosa porcorum | |
| Qualis vexillum | EP | |
| characteribus | Aspectus | albus vel fere albus;hygroscopic pulveris |
| solubility | gratis solutum in aqua | |
| idem | The13Spectrum C NMR adeptus est simile illi cum nadroparin calcii specificati CRS . | |
| Anti- factor Xa actio/ anti-factor IIa : 2.5-4.0 | ||
| Mw : 3600 -5000Da, M≤2000: NMT 15%, M2000-8000:75%-95%, M2000-4000:35%-55% | ||
| Prosequitur tium calcium | ||
| Solutio specie | Solutio non magis opalescentior quam relatio suspensionis II et non intensius coloratus quam relatio solutio Y5 . | |
| Ethanol | ≤1.0% | |
| pH | 5.5-8.0 | |
| N-NO coetuum | ≤ 0.25 ppm | |
| Free sulfates | ≤ 0.5% | |
| Ratio molaris sulfatis carboxylata | ≥1.8 | |
| Nitrogen | 1.5% -2.5% (ex exaruit basis) | |
| calcium | 9.5% -11.5% (ex exaruit basis) | |
| Damnum in siccitate | NMT 10.0% | |
| Bacterial endotoxins | <0.01eu / iu | |
| Assay | 95-130 iu / mg (ex arida basis) | |
Indicia
In chirurgia adhibetur ad praeventioni morborum venarum thromboembolicorum in casibus medii vel magni periculi venarum thrombosis.
Curatio venae altae thrombosis quae elaboravit.
Aspirin deducta ad tractationem angina pectoris instabilis et acuta periodus myocardialis infarctionis non-Q-undae.
Praeventionis sanguinis concreti formatio in cardiopulmonario bypass in hemodialysin.
CAS No.: 679809-58-6
CAS No. : 80449-31-6 (Trypsin Inhibitor Activity)